The EU defines photocatalysis as the activity that occurs when a light source interacts with the surface of semiconductor materials (called photocatalysts).
Photocatalysis is used, for example, on the International Space Station to break down pollutants and recycle the air that astronauts breathe.
How does photocatalysis work?
Briefly, photocatalysis is a radiation-driven phenomenon through which a substance known as a “photocatalyst” is activated by natural (solar) or artificial (ultraviolet) radiation to accelerate a chemical reaction. It can be compared to the chemical process of photosynthesis: the photocatalyst uses light energy, water and oxygen from the air, which causes the formation of highly reactive radicals. These radicals can break down specific organic and inorganic compounds in the atmosphere, turning them into oxidized compounds (for example, carbon dioxide and water). During this chemical reaction, the photocatalyst is not consumed or altered, which makes the process sustainable over time. Contrary to normal decontamination processes, the main products resulting from the photocatalytic reaction (CO2 and H2O) are harmless.
Numerous studies conducted on photocatalytic disinfection have shown that titanium dioxide, together with UV radiation, can be applied to inactivate microorganisms (for example, the SARS coronavirus).

Negative effects of photocatalysis (if used alone)
The downside of this technology, if used alone, is that it can also produce too high amounts of ozone (O3), a chemical variant of oxygen in the air that is itself a toxic pollutant.
When you hear the term “ozone” you may think of the planet’s ozone layer, which protects all life from the dangerous UV radiation of the sun. This is the “good” ozone that is present in the stratosphere. But when ozone exceeds certain limits at ground level is “bad” as it is hazardous to health and is classified as an air pollutant (ozone can cause a variety of health problems, like coughing and airway inflammation; it can also reduce lung function and harm lung tissue and certain groups like children, people with asthma, and older adults are especially vulnerable).
Proponents of photocatalysis-only processes claim that the amounts of ozone produced are within the limits suggested by the US FDA, WHO and other international bodies (0.05 parts per million). Although hydroxyl radicals occur naturally in the atmosphere, they themselves can present dangers: if the air being subjected to the process contains Volatile Organic Compounds (chemicals used in products such as paints and hairspray that evaporate easily) instead of being completely eliminated, they can become other pollutants, such as formaldehyde and acetaldehyde. Therefore, there is some debate and uncertainty about whether the pollutants produced during photocatalytic processes could even present a greater risk to human health than the risks to be eliminated.
UV rays are radiation with a wave frequency below the spectrum visible to the human eye and are characterized by having a high wavelength and, therefore, a high energy level. They are not seen, but they have important effects.
It is not a new technology since in the mid‑1930s, William Wells already demonstrated for the first time that UV rays could inactivate microorganisms suspended in the air. He later installed the technology in schools outside of Philadelphia to prevent the spread of measles. It was widely used in the 1950s and 1960s in healthcare settings and gained renewed attention during the drug-resistant tuberculosis outbreak in the U.S. It is widely used in other parts of the world such as Africa, Asia, and South America, where drug‑resistant tuberculosis is a particular problem.
What does UV-C radiation do to slow down pathogens?
It has been shown that the maximum absorption wavelength of a DNA molecule is, in the UV-C range, around 260nm. After UV-C irradiation, the DNA sequence of microorganisms’ form pyrimidine dimers, which interfere with DNA duplication, as well as destroy nucleic acids and cause viruses to cease to be infectious. And the effects are always similar for all viruses regardless of genome type.
Thus, UV-C radiation damages the DNA of microorganisms (bacteria, viruses, spores and other pathogens), destroying their ability to replicate and, therefore, making them non-infectious, as has been certified by the National Sanitation Foundation and the American National Standards Institute by classifying it as a disinfectant for reducing pathogen populations by at least a 3 log rate (99.9%).
How is UV-C radiation produced artificially?
Traditionally, UV-C radiation has been produced by means of continuous emission low and medium pressure mercury lamps. These lamps have the drawbacks that they are large and difficult to handle, they are not very efficient (high consumption), short duration, content of hazardous materials (mercury) and generation of high ozone concentrations (O3).
The new LED technology provides efficient irradiation at 275nm (close enough to 260nm that it has also been shown to cause significant effects on the DNA of bacteria and viruses).
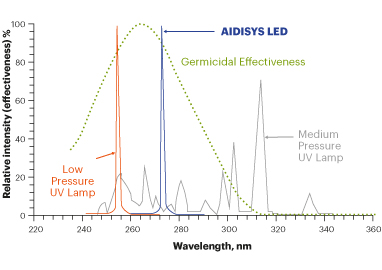
¿How does UV-C radiation affect humans?
Wavelengths shorter than 280nm are completely absorbed by our atmosphere and no natural UV-C radiation reaches the earth’s surface; therefore, the beings that inhabit the planet are not directly exposed to the effects of UV-C generated naturally.
In the case of humans, exposure to UV-C radiation (at least greater than 222nm) is directly associated with burns and skin cancer and eye damage such as photokeratitis (inflammation of the cornea of the eye), photo conjunctivitis (inflammation of the mucous membrane that lines the inner surface of the eyelids) and cataracts. Direct exposure without proper protective equipment should be avoided.
AIDISYS uses high‑performance, long‑lasting LED (275nm) technology to alter DNA from pathogens entering the system.
Furthermore, while UV radiation in the 160 – 240 nm range creates ozone from oxygen (by photolysis of O2), ultraviolet light wavelengths between 240-280nm will destroy ozone through photolysis of the ozone molecule.
Even without being activated, carbon, by its nature, is an excellent absorbent material thanks to the large number of pores that its structure contains. Activated carbon is much more porous carbon (a single gram has a surface area of more than 500m2) that traps compounds, mainly organic, present in a gas or a liquid.
Properties of activated carbon.
The more porous the coal, the more pollutants it will capture. The more activated carbon in a filter, the more contaminants it will retain and the faster it will adsorb them. Also, high levels of activated carbon increase filter life (requiring fewer replacements).
How does an activated carbon filter work?
The carbon atoms that make up the solid called “carbon” are linked together by covalent bonds. Each atom shares an electron with four other carbon atoms.
The atoms that are not on the surface, distribute their four bonds in all directions. But the surface atoms, although they are linked with four others, are forced to do so in less space, and an imbalance of forces remains in them.
This imbalance is what allows them to trap a molecule of the fluid that surrounds the coal.
The molecules that carbon adsorbs tend to be covalent, and since the bonds between carbon and hydrogen atoms are covalent, carbon is a good adsorbent for organic molecules.
Precautions for use.
Coal tends to compact when the relative humidity of the environment is quite high (above 75%). This effect would prevent the air from passing through the coal itself, making its filtering more difficult and causing the air circulation within the system to suffer flow retention.
Technologies used in AIDISYS products
UV-C irradiation is highly effective in inactivating and inhibiting SARS-CoV-2 replication (scientific publication)
The effect of 222-nm UVC phototesting on healthy volunteer skin: a pilot study that concludes that this frequency also produces erythema and cyclobutane pyrimidine dimers (CPD) formation in human skin (scientific publication)
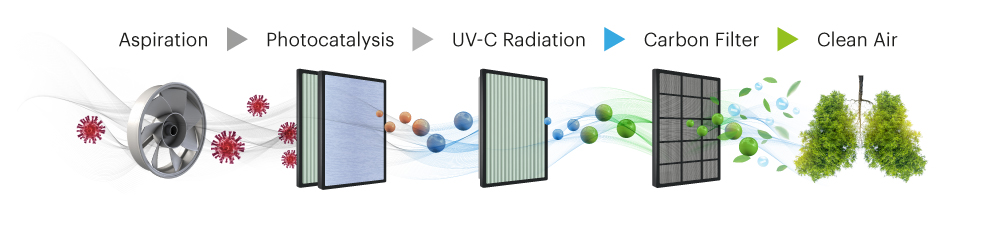
Aspiration
Using extractors, the air in the room is introduced into the AIRISYS disinfection and purification system. A pre‑filter retains larger elements such as hair, insects, etc.
Photocatalysis
Latest generation high power UV-A LEDs activate a titanium dioxide filter that, together with oxygen and water in the environment, produce radicals that neutralize organic and inorganic compounds, turning them into oxidized compounds.
UV-C Radiation
The latest generation high power UV-C LEDs also inactivate specific organic and inorganic compounds in the atmosphere and remove ozone and other gases generated in photocatalysis.
Carbon Filter
High porosity activated carbon filter that traps compounds, mainly organic, that are in the atmosphere and allows to retain particles and odors.
Clean Air
Once pollution has been eliminated, the clean air is reverted to the environment and can be breathe by the beings in the room without harmful particles and organisms be entering their body.
